|
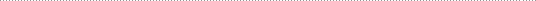
Covid-19
Virus

TRIPHASE® PharmaSolutions,
LLC, provides drug development services in
chemical engineering, chemistry, manufacturing, and controls (CMC) for drug
substance,
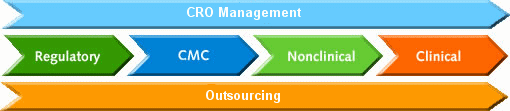
analytical, pre-formulation, formulation, salt &
polymorph selection, cGMP/GXP manufacture of toxicology and clinical supplies,
stability, impurities, specifications, pre-IND and EU
Scientific Advisory Meeting regulatory filings
to support an IND, IMPD, NDA, 505b2, or ANDA in
CTD format. Therapeutic areas of expertise include
dermatology, ophthalmology, cardiology, and oncology.
Triphase is seeking partners in these areas to develop drugs under a consultancy
(partnering)
model. From the inception of your project to submission we
provide the essential scientific and regulatory leadership to manage
your program in all areas such as toxicology drug
supplies, clinical drug supplies, analytical validations, formulation
of oral solid or liquid dosage forms, sterile liquids for
ophthalmic or injectable use, lyophilized products, manufacturing
compliant to GMP, ICH, ISO, QA/QC audit and oversight,
stability study design and problem solving (impurities), specifications, and
consolidation all your data into a coherent regulatory
filing
to obtain your approvable letter. Please
learn more by reading about our services or contact us
directly at info@triphasepharmasolutions.com.
.
DOWNLOAD
SERVICE BROCHURE HERE
|

